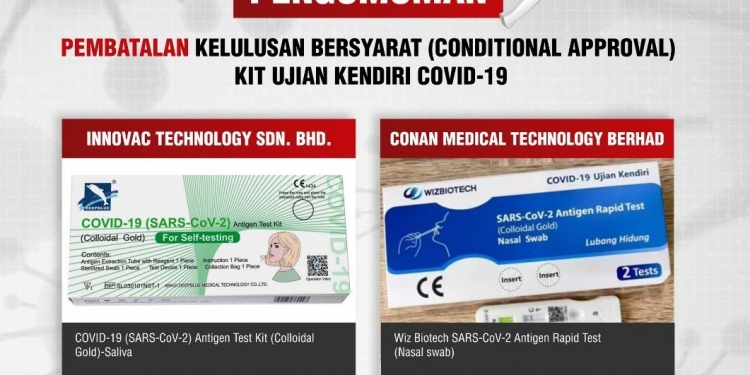
The Medical Device Authority (MDA), under the Ministry of Health, has withdrawn the conditional approval for two COVID-19 self-test kits. The revocation takes into effect immediately due to the failure to comply with the conditional approval terms granted by the MDA.

The two revoked COVID-19 self-test kits are COVID-19 (SARS-COV-2) Antigen Test Kit (Colloidal Gold)-Saliva manufactured by Anhui Deepblue Medical Technology, Co, and Wiz Biotech SARS-CoV-2 Antigen Rapid Test (Nasal swab) by Xiamen Wiz Biotech Co. Ltd. Both are manufactured in the People’s Republic of China and are distributed by Innovac Technology Sdn Bhd and Conan Medical Technology Berhad respectively.
Consumers are warned not to buy or use the above test kits as they are deemed unsafe to be used. As of 8th April 2022, the Medical Device Authority has given conditional approval to 128 COVID-19 self-test kits. You can view the current approved list here.
Back in February, Health Minister Khairy Jamaluddin called upon the MDA to address accuracy concerns of COVID-19 self-test kits in the country. He said checks on the quality assurance process by suppliers from manufacturers must be made for each self-test kit from time to time. He shared concerns that when the kits were approved, the accuracy might be 99% but the rate may decline over a period of time.
Saya telah menerima beberapa aduan berkenaan kit ujian kendiri COVID-19 yang ada di pasaran. MDA telah menguji 22 jenama. Setakat ini, 12 keputusan telah diperolehi dan mendapati 2 kit tidak mencapai tahap prestasi yang ditetapkan. Kelulusan 2 kit ini digantung serta-merta. pic.twitter.com/svwbHNRcKv
— Khairy Jamaluddin 🇲🇾🌺 (@Khairykj) April 11, 2022
According to Khairy this evening, MDA has recently tested 22 brands following complaints made by the public. Out of 12 test results received so far, two have failed to achieve acceptable performance levels. As a result, the conditional approvals granted previously were revoked immediately.
The folks at Cilisos have evaluated a bunch of COVID-19 test kits with a COVID-19 positive person and discovered that 2 out of 10 kits had failed. They have conducted another round of tests again with one of their writers who got COVID-19. This time, only two out of 10 kits were showing positive, while the rest had a negative result during Day 5 of showing symptoms.
It is worth highlighting that Rapid Test Kits (RTK-Antigen) are only effective during your most infectious period. The RT-PCR (Real-Time Reverse Transcription Polymerase Chain Reaction) is still the gold standard in COVID-19 detection. However, RT-PCR is more expensive and must be conducted at a healthcare facility or lab.
[ SOURCE ]