Malaysia has just received its first batch of Sinovac vaccines from China. Unlike the Pfizer vaccines which arrived as a finished product, the Sinovac vaccines are ordered in bulk and it will undergo a local “fill and finish” (F&F) process.

The Chinese vaccines were transported in a temperature-controlled container on Malaysia Airlines flight MH319. The Airbus A330 departed Beijing at 3.06am and it arrived at KLIA at 8.50am this morning.
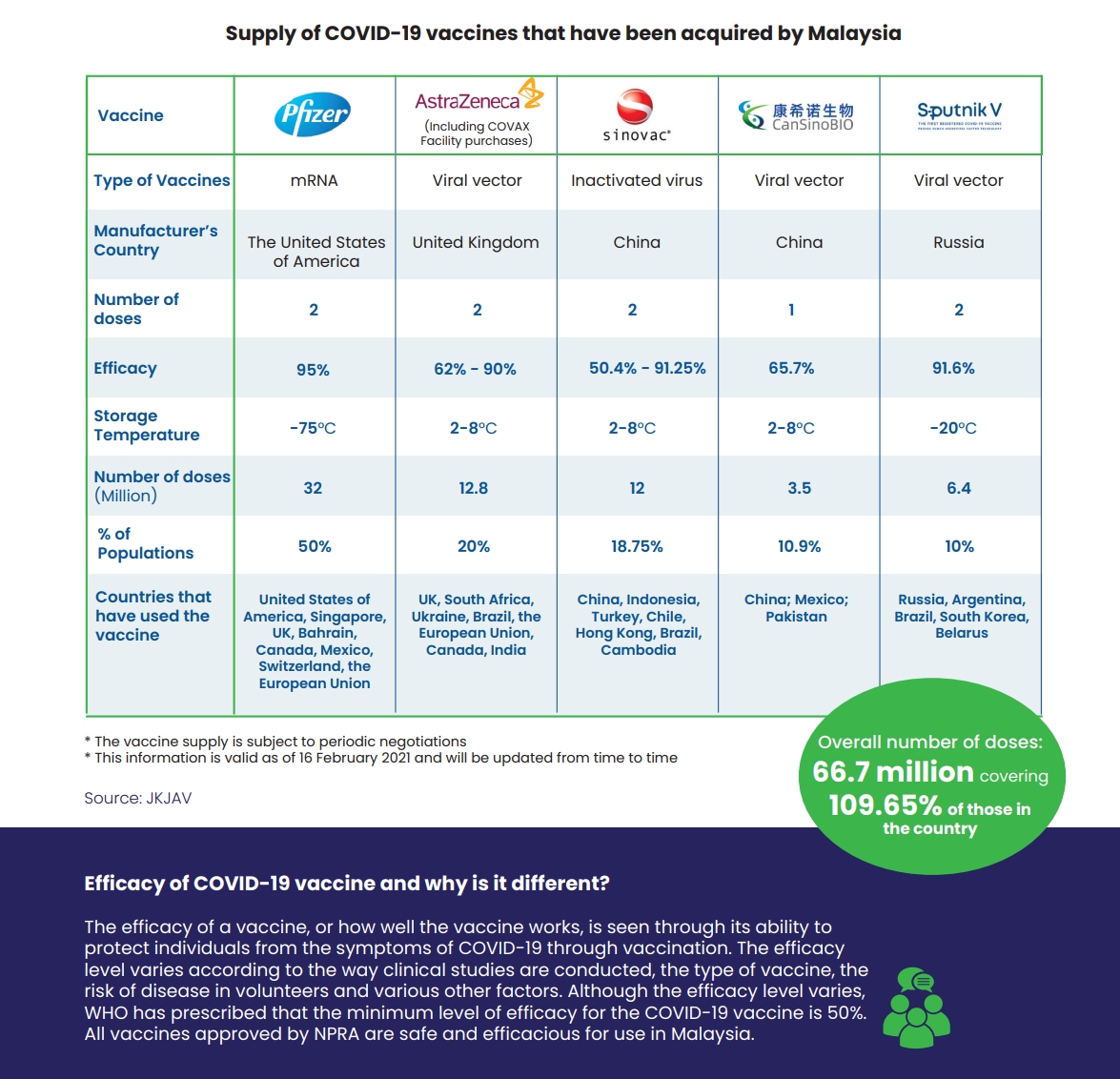
For the first batch, Malaysia gets 200-litres in bulk which is enough to provide about 315,000 doses. Sinovac vaccines are developed with inactivated virus, which uses a virus that has been killed to stimulate the human immune response.
Unlike the mRNA-based Pfizer vaccines, Sinovac doesn’t require extreme cold storage and it can be stored at 2-8°C. Two doses will be required for each individual and Malaysia has procured 12 million doses, which is enough to cover 6 million people. Sinovac vaccines are deemed halal by the Indonesian authorities.
Sinovac vaccines are pending approval
At the moment, Pfizer-BioNTech vaccine is the only option that has received conditional approval from the Drug Control Authority (DCA) and the National Pharmaceutical Regulatory Agency (NPRA). The remaining vaccines including AstraZeneca, Sinovac, CanSinoBio and Sputnik V are still pending approval from the NPRA.
To bring the cost down, the Sinovac vaccines that are purchased in bulk will undergo a F&F process at Pharmaniaga’s facility in Puchong. The process involves filling vials with the vaccine and finishing the process of packaging for distribution.
Before it can be administered, the vaccines will require two approvals from the NPRA. This include approval for the vaccine itself and also the approval for the F&F process by Pharmaniaga.
Once it has obtained approval, Pharmaniaga targets to release 2 million doses of the Sinovac vaccine each month. The efficacy rate of Sinovac vaccine is 50.4-91.25%.
What happens if NRPA doesn’t approve the Sinovac vaccine?
Some have questioned why are we getting vaccines that are not yet approved. Minister of Science, Technology and Innovation, Khairy Jamaluddin explained that the approval will also need to cover the local bottling process by Pharmaniaga.
The NPRA will perform a Good Manufacturing Practice (GMP) inspection to verify Pharmaniaga’s compliance and ensure that the vaccines produced are of the quality required for its intended use. According to Pharmaniaga Group Managing Director Datuk Zulkarnain Md Eusope, the F&F facility and full manufacturing plant isn’t just the first in Malaysia, but it’s also the first halal vaccine plant in the world.
So what happens if the NPRA doesn’t approve the vaccine? Under the agreement, Malaysia will not pay for the vaccines if it failed to obtain NPRA approval and it won’t be used in the immunisation programme.
As highlighted by Khairy, Singapore has also received the Sinovac vaccines while it is still pending approval from the local regulatory. Unlike Malaysia, Singapore is getting a finished product.
Responding to critics that Ministers and politicians are getting Pfizer vaccines which has a higher efficacy rate, Khairy has promised that he won’t be taking the same vaccine. Instead, he will be the first in line to receive the next approved vaccine by the NPRA. This could be Sinovac, Sputnik, Astra Zeneca or CanSino. This is to instill confidence in the NPRA’s diligence in ensuring that the approved vaccines are safe for use.
Menteri Penyelaras Program Imunisasi COVID-19 Kebangsaan, @Khairykj umum jadi individu pertama ambil vaksin yang akan diluluskan seterusnya.#MyVaksinCOVID19 #HapusCOVID19 pic.twitter.com/xIFcAOm6sr
— Astro AWANI (@501Awani) February 27, 2021
How to register?
In case you missed it, registration for the COVID-19 vaccination program in Malaysia is now open via MySejahtera which is available on Google Play Store, Apple App Store and Huawei AppGallery. For those without access to the app, you can register via the website and hotline phone number which will be available from 5th March 2021.
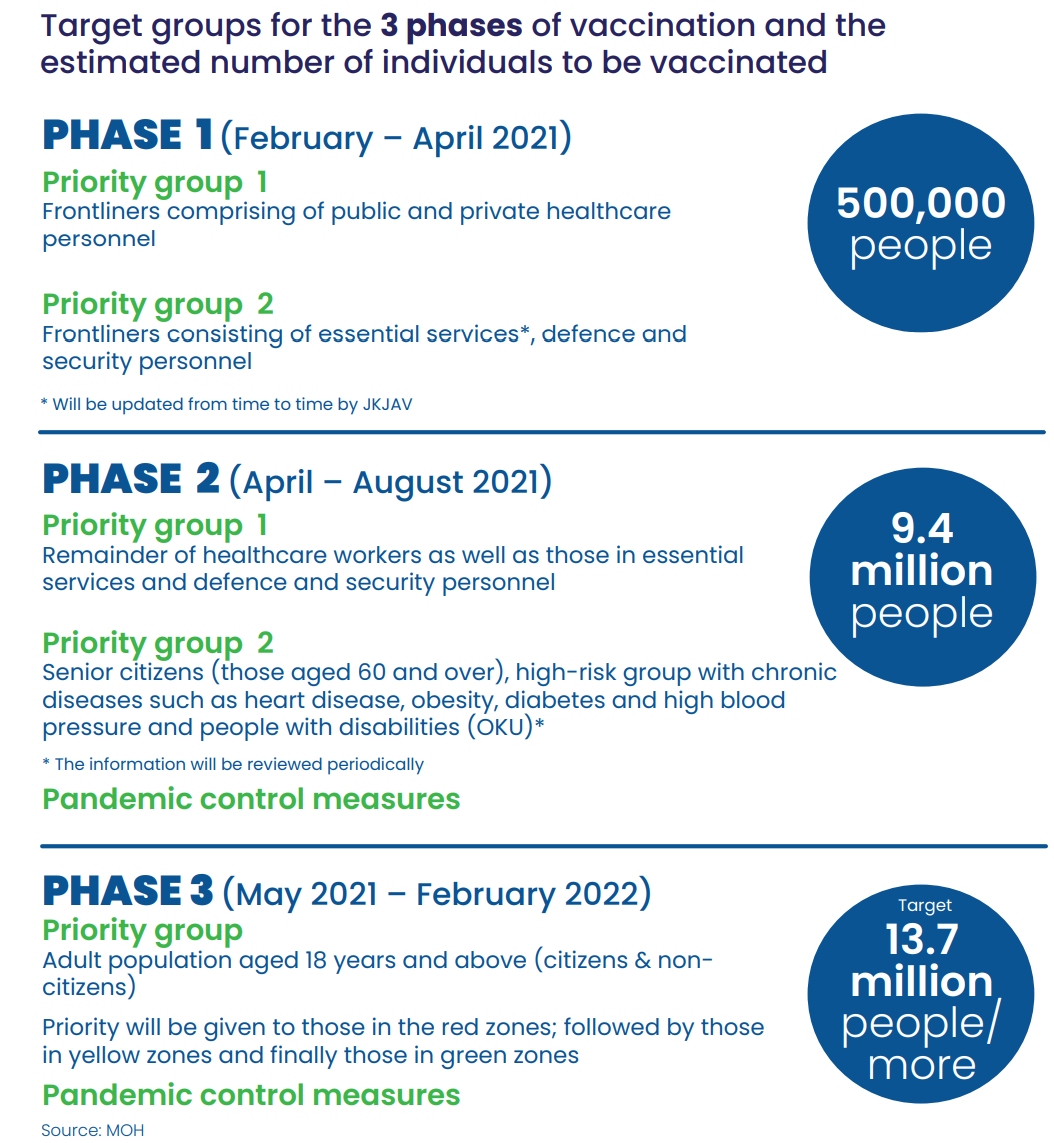
The vaccination is offered to free to everyone including both citizens and non-citizens in Malaysia. The programme is currently in Phase 1 which will cover 500,000 frontliners and essential workers. Malaysia aims to vaccinate everyone by the end of this year, provided that the vaccines are approved and the supplies can be delivered according to schedule.
For further reading, you can refer to the Special Committee for Ensuring Access to COVID-19 vaccine supply (JKJAV) website.
[ IMAGE SOURCE ]










