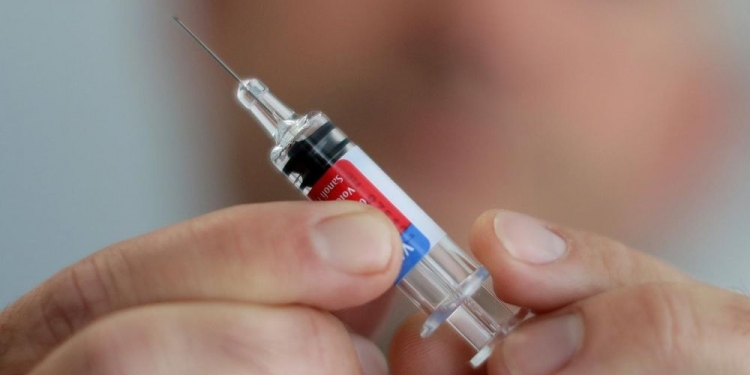
Biotechnology company Moderna claims that their COVID-19 vaccine candidate is 94.5 percent effective, based on early data. That’s slightly higher than the 90 percent efficacy of a vaccine by Pfizer and BioNTech—which was announced last week.
“Aspirationally, you would like to see 90, 95 percent, but I wasn’t expecting it. I thought we’d be good, but 94.5 percent is very impressive,” Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases.
While these efficacy readings are higher than many experts were expecting, the data hasn’t yet been published or reviewed by outside experts. Moderna’s initial evaluation included 90 cases of COVID-19 in the clinical trial group that received the placebo, and only five cases in the group that were given a vaccine. In the trial, they found 11 severe cases in the placebo group, but none in the group receiving the vaccine.
For the vaccine trial results by Pfizer and BioNTech, they included 94 cases of COVID-19. Fewer than nine of those cases were among people who received two shots of their vaccine—which resulted in their vaccine being 90% effective.
Moderna also announced that its COVID-19 vaccine can be stored at refrigerator temperatures (between 2 to 8 degrees Celsius) for up to 30 days—which should help make storage and distribution of this vaccine easier. Pfizer and BioNTech’s vaccine has to stay at -70 degrees Celsius.
“By the end of 2020, the Company expects to have approximately 20 million doses of mRNA-1273 (their vaccine) ready to ship in the U.S. The Company remains on track to manufacture 500 million to 1 billion doses globally in 2021,” wrote Moderna.
Recently, Microsoft reported that COVID-19 vaccine makers are under attack by Russian and North Korean hackers. The attacks aimed at seven pharmaceutical companies and researchers in the U.S., Canada, France, India, and South Korea.
[ SOURCE, IMAGE SOURCE ]